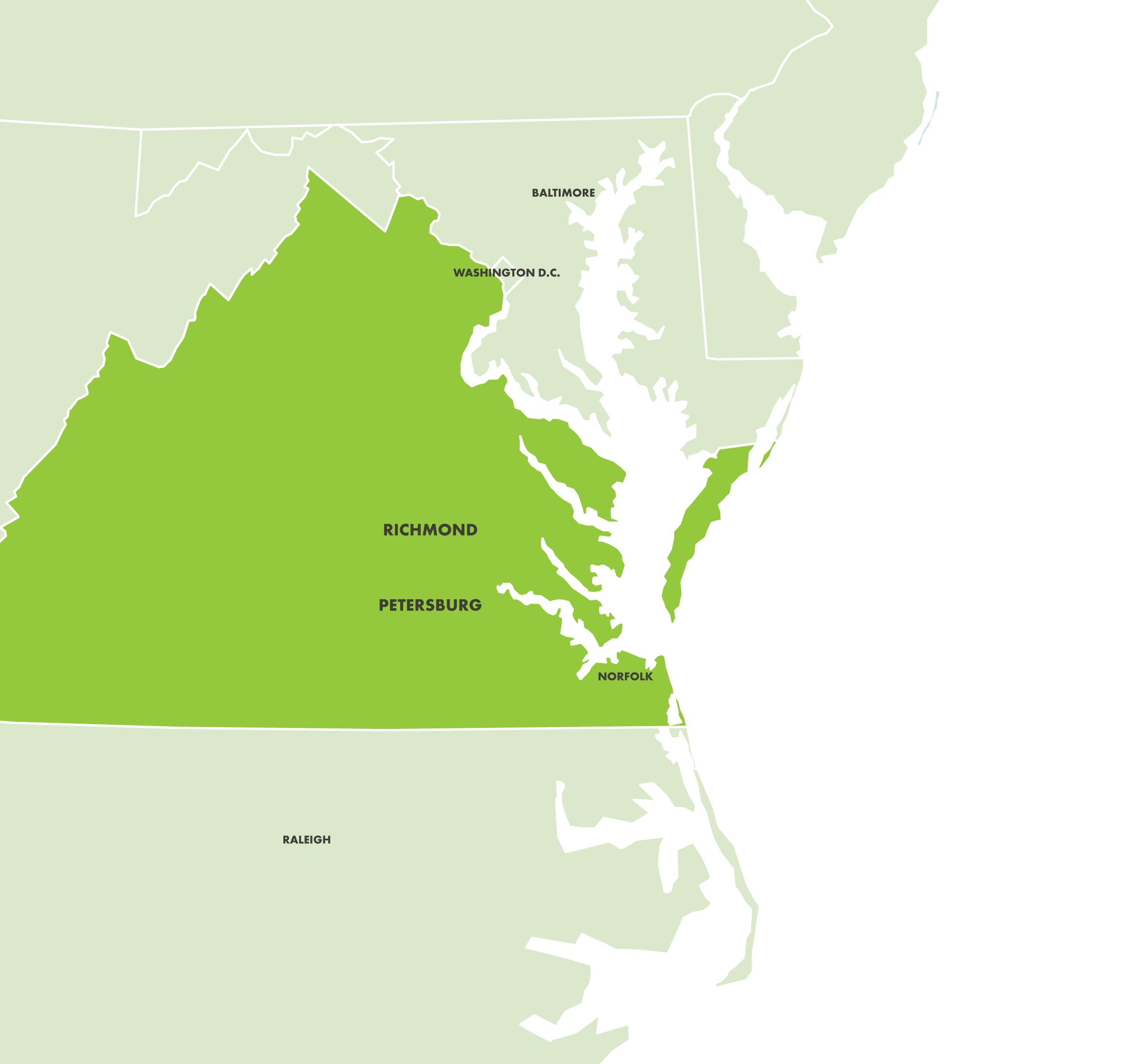
Built for Supply Chain Resiliency and Reliability.
Phlow’s U.S.-based manufacturing infrastructure aims to address the structural vulnerabilities in international supply chains and safeguard America’s economic and national security.
Phlow's Research & Development Laboratory
Phlow Corporate Headquarters
Petersburg Campus
Kilo-Scale cGMP Facility
Metric Ton-Scale cGMP Facility
Phlow’s Advanced Manufacturing Facilities.
Phlow’s Research & Development Laboratory.
Phlow’s state-of-the-art R&D lab is located at Virginia Biotechnology Research Park in Richmond, Virginia.
Phlow offers R&D services for small molecule Active Pharmaceutical Ingredients (APIs) and Key Starting Materials (KSMs) that leverage advanced manufacturing technologies using the latest process and analytical equipment. Here we focus on initial route scouting and process development, coupled with industry leading analytical capabilities.
Located in Richmond, VA

Located in Petersburg, VA
Kilo-Scale cGMP Facility.
Phlow’s Kilo-Scale cGMP Facility is an advanced manufacturing facility capable of both batch and continuous processes for producing up to 500 kg of API annually. It features highly automated custom-built skids, 100 L reactors spread across two cGMP suites, and additional non-cGMP and R&D lab space with 12 fume hoods, analytical & R&D support, and an automation lab.
Metric Ton-Scale cGMP Facility.
Phlow’s Metric Ton-Scale cGMP Facility can produce 60 metric tons of API annually, through continuous and batch mode production. This large-scale, advanced manufacturing facility is equipped with multiple production lines and dedicated drying suites.
Located in Petersburg, VA
Phlow’s Research & Development Laboratory
CHEMICAL DEVELOPMENT
PROCESS SAFETY STUDIES
PROCESS AND ANALYTICAL EQUIPMENT
- Fluorination
- Hydrogenation
- Cyanation
- Azidation
- Cryogenic Chemistry
- Methylating Agents
- Chromatography
- Heterogeneous/Homogeneous Catalysis
- Nitrations
- Metal-Mediated Couplings
- Alkylations
- Acylations
- Nucleophilic Aromatic Substitution Reactions
- Pyrophoric Reagents
- Synthesis of Reference Standard Samples
- Organic Chemical Process Development
- Advanced technology: Biocatalysis
PROCESS SAFETY STUDIES
- Process Safety Investigations
- Calorimetric Measurements
PROCESS AND ANALYTICAL EQUIPMENT
- EasyMax 402
- Jacketed Reactors
- Various Sized CSTRs
- Various Pumps
- Mass Flow Meters
- IR/Raman with Probe
- LCMS – Triple-Quadrupole
- LCMS – QToF
- GC-Mass Spectrometer
- HPLCs/UPLCs
- Benchtop 80MHz NMR w/ Flow Cell
- Phoenix Flow Reactor
- H-Cube Systems
- Corning Advanced-Flow Reactor
- Differential Scanning Calorimeter
- Hydrogenation Equipment
Kilo-Scale cGMP Facility
ANNUAL CAPACITY OF 500 KG
- 2 cGMP suites & 1 non-cGMP suite
- 2 in each of 2 cGMP Kilo rooms
- 2 in the Continuous Lab (non-cGMP)
- 6 Support Tanks for each cGMP Hood (24 total)
- Hastelloy and 316 L SS
- 10 gal and 20 gal
- Agitated and Jacketed
CAPABILITIES
Capable of producing quantities of API in the range of up to ~2 kg/day, depending upon the nature and complexity of the API process.
Metric Ton-Scale Facility
ANNUAL CAPACITY 60 MT
EQUIPMENT
- Eight reactors in a single reactor suite
- Dryer/centrifuge charge & discharge rooms
- Filter/dryer charge and discharge rooms
- 2 Process Equipment Trains, 4 Reactors per Train
- 1,000 gal reactor, 2,000 gal reactors
- Hastelloy
- Glass-lined Steel
- 2,000 gal Hastelloy Filter Reactor (custom design)
- Transfer Booths
- Liquid booths – Level 1
- Solids booths – Level 2
- Filtration & Drying
- Filter Dryer
- Inverting Basket Centrifuge
- Conical Dryer
Download the Brochure
Explore how Phlow’s state-of-the-art manufacturing infrastructure can revolutionize your small molecule API development and production.